about premstem
research activity overview
project activities
PREMSTEM is a research project in the field of regenerative medicine which has received funding from the European Union’s Horizon 2020 research and innovation programme. The following is an overview of our planned activities:
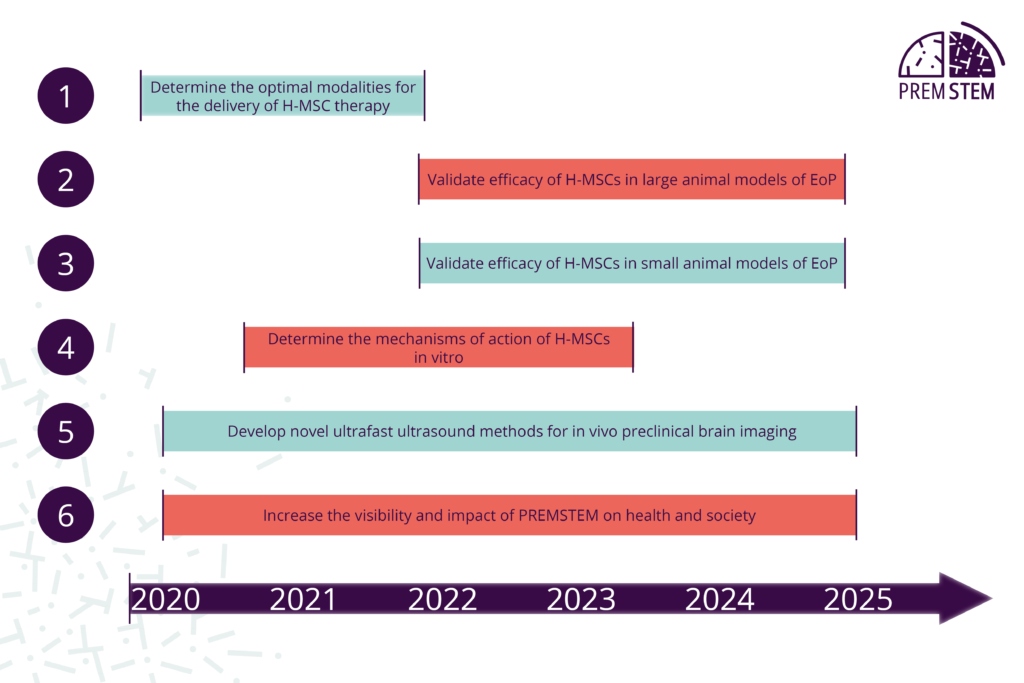
- The efficiency of a given therapy heavily depends upon its delivery modalities. Our first step is to determine the best dose, time and delivery route to get a therapeutic effect of H-MSCs in rodent models of encephalopathy of prematurity (EoP).
- Then, using large animal models of EoP that capture important hallmarks of the human pathology, our objective is to study short-term as well as long-term effects of H-MSC induced-neuroregeneration, especially on motor behaviour and cognitive function.
- In addition, another part of our research is dedicated to gather even more knowledge about the therapeutic effects of H-MSCs on neuronal and vascular development, inflammatory responses and cellular metabolism, to name a few, using our multiple and clinically relevant rat models of EoP.
- In parallel, understanding the mechanisms of action of H-MSCs at the molecular and cellular levels is crucial to determine which babies with EoP will most benefit from H-MSC therapy. This part is addressed with our numerous in vitro models.
- Technological innovation enabling better characterisation of preterm infants and its transfer to neonatologist clinicians is one of the main objectives of PREMSTEM. Developing a novel imaging platform to meet the needs of clinicians in future identification of preterm babies based on injury type and severity is a fully integrated part of our activities.
- With 15 million infants born preterm every year worldwide, bringing our research into improved human health outcomes is our major objective. Engaging key stakeholders in the neonatal ecosystem (patients/consumers representatives, clinicians and care givers, scientific community, policy makers, the general public) and consulting them on experimental design and implementation is a crucial step to fast track the use of H-MSCs to bedside clinical research, and ultimately to improve health outcomes for babies.
PREMSTEM is led by an interdisciplinary consortium of world leading clinicians, researchers, stakeholder advocacy groups and an industrial partner across eight countries. All PREMSTEM partners conform to current legislation and regulations in the countries where the project’s activities are carried out. PREMSTEM investigators and their staff conduct research studies in adherence to the fundamental ethical principles applicable when conducting the studies.